GC Cell announced on January 30 that it presented its first-in-class CD5-targeting allogeneic cord blood-derived Chimeric Antigen Receptors-Natural Killer (CAR-NK) cell therapy, GCC2005, at the 17th T Cell Lymphoma Forum (TCLF) in San Diego, U.S.
TCLF is a specialized conference where global experts in T-cell lymphoma convene to discuss cutting-edge research findings, diagnostic techniques, and treatment strategies. Unlike broader hematology conferences, TCLF facilitates in-depth discussions among specialists focused on this particular disease.
GCC2005 has now been featured in oral presentations at both the 67th American Society of Hematology (ASH) meeting and TCLF, underscoring its clinical potential and technological edge as a treatment for relapsed or refractory T-cell lymphoma.
Dr. Kim Won-seok from Samsung Medical Center’s Department of Hematology and Oncology delivered the presentation. He discussed the development of the CD5 CAR-NK cell therapy for peripheral T-cell lymphoma during a session exploring the role of adoptive immunotherapy in treating this condition.
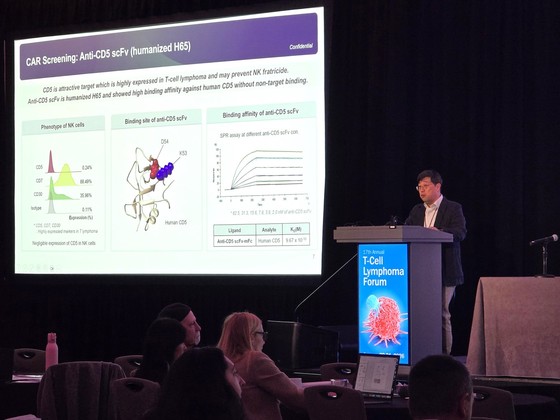
The presentation outlined GCC2005’s development journey, from initial research stages to its current clinical phase. It included data on the rationale behind the optimal CAR structure selection, as well as in vitro and in vivo characterization data, demonstrating GC Cell’s scientific prowess.
Notably, the presentation highlighted experimental results showing significantly reduced risks of fratricide and T-cell aplasia, long-standing challenges associated with existing autologous CAR-T therapies. This breakthrough garnered considerable interest from the global scientific community.
The presentation also shared interim results from a Phase 1 clinical trial conducted in Korea, previously disclosed at ASH. The Phase 1a interim analysis revealed that among nine patients with CD5-positive relapsed or refractory NK/T-cell lymphoma, no dose-limiting toxicities (DLT) or serious adverse events were observed. Importantly, the infection issues reported with competing CD5 CAR-T therapies were not seen.
Efficacy results were promising, with an objective response rate (ORR) of 62.5% among eight evaluable patients. This included three patients achieving complete response (CR) and two achieving partial response (PR). These figures are particularly encouraging, given that typical response rates for existing chemotherapy agents in this patient population are below 30%.
One patient achieved complete response after just a single administration, further supporting GCC2005’s antitumor potential. Response rates tended to improve with increased dosage, and even some patients with progressive disease showed reductions in target lesions.
A compelling case study of a patient with angioimmunoblastic T-cell lymphoma (AITL) was presented. This patient, who had failed three previous treatments, maintained complete response for six months after receiving treatment in the low-dose group. The patient is currently in the ninth month of follow-up.
GC Cell is currently conducting a dose-escalation study for high-dose administration of GCC2005. The company plans to expand development through a domestic Phase 1b trial and a global Phase 2 trial for further dose expansion studies.
GC Cell Chief Executive Officer (CEO) Won Seong-yong stated that they are honored to share GCC2005’s achievements at TCLF, a gathering of world-renowned experts. The high expectations observed at both ASH and TCLF motivate them to expedite its remaining clinical procedures and advance discussions for global partnerships. The ultimate goal is to provide this innovative treatment option to patients worldwide.
