Top News
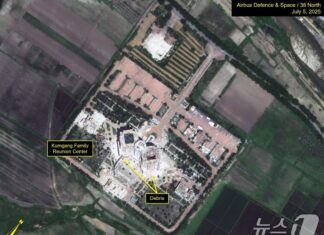
North Korea Is Tearing Down South Korean Resorts—But What Comes Next?
North Korea is dismantling South Korean facilities at Mount Kumgang, but redevelopment plans remain unclear despite potential UNESCO listing.
- Our Sponsors Ad -