
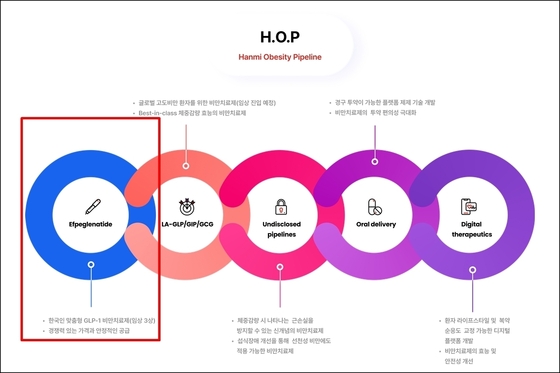
Hanmi Pharmaceutical is launching its comprehensive Hanmi Obesity Pipeline (H.O.P) project, aiming to revolutionize the entire obesity treatment process. Leveraging its expertise in drug development, the company is set to make waves in the global obesity market with a diverse portfolio. This includes GLP-1 formulations tailored for Korean body types, innovative drugs tackling muscle loss during weight reduction, and cutting-edge digital solutions.
Industry reports from Wednesday indicate that Hanmi Pharmaceutical has achieved a significant milestone. The company has secured top-line results from completed Phase 3 clinical trials for its flagship product, efpeglenatide, and is now navigating the product approval process.
Efpeglenatide, a once-weekly GLP-1 receptor agonist, utilizes Hanmi’s proprietary Labscov platform technology. Clinical trials have demonstrated its superior weight loss effects compared to placebo. Notably, the drug’s dosage is optimized for Korean patients, who typically have lower BMIs than their Western counterparts, minimizing side effects.
While other GLP-1 products from global pharmaceutical giants often cause gastrointestinal side effects like vomiting and nausea, efpeglenatide has shown a reduced incidence of such adverse events.
Hanmi Pharmaceutical has confirmed efpeglenatide’s efficacy in providing stable blood sugar control and promoting weight loss. Industry experts predict that upon market entry, efpeglenatide will swiftly replace existing obesity treatments, with commercialization expected in the latter half of this year.
HM15275, Hanmi’s Next-Generation Triple-Action Drug, Making Steady Progress in Global Clinical Trials
While efpeglenatide targets the domestic market, HM15275 sets its sights on the global stage. This innovative drug simultaneously acts on three receptors: GLP-1, gastric inhibitory polypeptide (GIP), and glucagon.
HM15275 is designed to maximize weight loss by combining the appetite-suppressing and insulin-promoting effects of GLP-1 and GIP with glucagon’s role in boosting energy metabolism.
Hanmi Pharmaceutical aims to develop HM15275 to achieve over 25% weight loss, surpassing the effects of gastric bypass surgery. The drug is currently undergoing research with the goal of entering Phase 2 clinical trials in the U.S. and other global markets.
If clinical trials prove successful, HM15275 is poised to offer a groundbreaking treatment option for severe obesity patients, potentially overcoming the weight loss limitations of current obesity treatments.

Burns Fat, Keeps Muscle: Novel Injectable Meets Digital Innovation
Hanmi Pharmaceutical is pushing the boundaries of obesity treatment with new drug candidates designed to bring about qualitative changes, going beyond mere weight loss. A key player in their pipeline is the innovative obesity treatment HM17321.
Current GLP-1 drugs have a significant drawback: they reduce both fat and muscle mass during weight loss, leading to decreased basal metabolic rates and potential weight rebound.
HM17321 takes a different approach by targeting the CRF2 receptor, which inhibits muscle breakdown while promoting fat loss. This novel mechanism allows for weight reduction while preserving or even increasing muscle mass, addressing the market’s demand for healthier weight loss solutions.
Hanmi is also developing HM101460, an oral GLP-1 obesity treatment based on next-generation, low molecular weight compounds. This drug aims to achieve superior efficacy and stability compared to existing injectable treatments through a G-protein biased activation mechanism. Hanmi showcased its potential by presenting preclinical results at the European Association for the Study of Diabetes (EASD).
In parallel, Hanmi Pharmaceutical is developing digital solutions to enhance and sustain the effects of drug therapy. The company is collaborating with digital healthcare firms to create digital therapeutic products that help patients improve their lifestyles and medication adherence.
This comprehensive strategy aims to prevent post-treatment weight rebound and provide a holistic solution for managing obesity as a chronic condition.
An industry insider commented that Hanmi Pharmaceutical’s H.O.P project is a game-changer, going beyond mere replication of obesity drugs. It strategically addresses market needs by developing formulations tailored for Koreans, tackling muscle loss, and integrating digital solutions. They added that the successful commercialization of efpeglenatide could kickstart a virtuous cycle, accelerating the development of future drugs with the generated revenue.
