HK inno.N’s P-CAB class drug K-Cap for gastroesophageal reflux disease has officially entered the approval process in the U.S., the world’s largest pharmaceutical market.
HK inno.N announced on Tuesday that Braintree, a subsidiary of Sebela Pharmaceuticals specializing in gastrointestinal medicines, submitted a New Drug Application (NDA) for K-Cap to the U.S. Food and Drug Administration (FDA) on January 9.
The application seeks simultaneous approval for three indications: treatment of non-erosive reflux disease (NERD), treatment of erosive esophagitis (EE), and maintenance therapy following EE treatment.
The application is supported by superior clinical data from a pivotal Phase 3 trial involving over 2,000 U.S. patients. Results showed that Tegoprazan, a P-CAB class drug, outperformed existing proton pump inhibitors (PPI) drugs across multiple endpoints. All metrics were analyzed using a pre-defined hierarchical multiple testing procedure.
In NERD patients, K-Cap demonstrated superiority over placebo in the proportion of 24-hour heartburn-free days. It also showed better results in nighttime heartburn-free days and acid reflux symptom-free days.
For all grades of erosive esophagitis, K-Cap significantly outperformed the PPI drug lansoprazole at both 2 and 8 weeks. Notably, it proved superior even in severe cases, highlighting its unique value in treating advanced conditions.
In a 24-week maintenance study following EE healing, K-Cap showed superior healing maintenance across all patient groups compared to PPI drugs. It was particularly effective in maintaining healing and relieving heartburn in severe cases.
Sebela plans to present the complete Phase 3 results at major conferences this year and publish the findings in leading medical journals.
HK inno.N Chief Executive Officer (CEO) Kwak Dal-won expressed enthusiasm, stating that they’re thrilled that K-Cap, a South Korean innovation, is advancing through the U.S. approval process with such promising clinical results. It is committed to establishing it as a best-in-class global treatment, with plans to expand into European and Japanese markets.
Alan Cooke, CEO of Sebela Pharmaceuticals, noted that in the U.S., 35% to 54% of the roughly 65 million gastroesophageal reflux disease (GERD) patients find inadequate relief with current treatments. Tegoprazan has shown superior sustained healing in severe EE cases compared to PPIs, and significant improvements in 24-hour heartburn, nighttime heartburn, and acid reflux symptoms in NERD patients.
Cooke added that it is collaborating closely with the FDA, aiming to bring Tegoprazan to patients and healthcare providers by January 2027.
Dr. Philip O. Katz of Weill Cornell Medical College commented that the introduction of P-CABs like Tegoprazan, offering faster onset and sustained acid control, marks a significant advance in GERD treatment. The Phase 3 data suggests Tegoprazan could improve symptom control in NERD and potentially boost healing rates in severe EE.
He added that P-CABs like Tegoprazan may help bridge the treatment gap for patients who continue to struggle despite PPI therapy.
K-Cap, South Korea’s 30th novel drug developed by HK inno.N, contains Tegoprazan as its active ingredient. This P-CAB class medication has demonstrated superior efficacy over PPI drugs in onset of action, duration of effect, and healing/maintenance of erosive esophagitis.
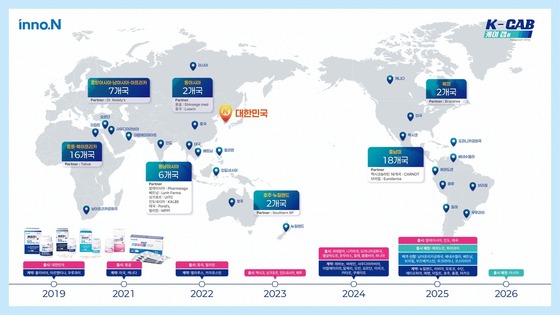
Since its domestic launch in March 2019, K-Cap has generated cumulative off-label prescription sales of 923.3 billion KRW (approximately 626.5 million USD), leading the market for digestive ulcer medications in South Korea. The company has secured technology or finished product export agreements with 55 countries, with approvals in 22 countries and launches in 19, including South Korea.
