On Tuesday, GI Innovation announced that it is ramping up development of a next-generation allergy treatment optimized using artificial intelligence (AI) technology.
The company’s novel dual fusion pipeline is designed to modulate the IL-4/IL-13 signaling pathway involved in allergic inflammation while simultaneously targeting the complex underlying causes of atopic dermatitis. Due to competitive considerations, GI Innovation has opted not to disclose specifics about mechanisms beyond that of Dupixent.
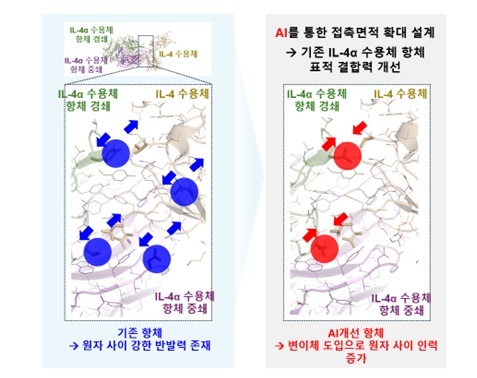
The company initiated this pipeline over three years ago, beginning with the lead candidate GI-305, and has since made continuous structural improvements and functional validations. Notably, it has focused on enhancing antibody binding affinity, building on the same mechanism as Regeneron’s blockbuster drug Dupixent, which generates annual sales of approximately $15 billion.
Recently, GI Innovation leveraged AI-based protein simulation technology to develop dual fusion candidates that balance structural stability and functional efficacy.
While Regeneron’s Dupixent currently dominates the atopic dermatitis market, GI Innovation’s newly patented compound is designed to simultaneously target multiple disease pathways and deliver a higher dose to affected areas.
This approach aims to overcome the limitations of existing systemic treatments by more precisely targeting affected sites, potentially offering advantages over current therapies.
The company is also co-developing GI-301 (YH35324) with Yuhan Corporation for chronic spontaneous urticaria, a market currently led by Xolair. Meanwhile, the new dual fusion candidate is being developed specifically to compete with Dupixent in atopic dermatitis.
In August of last year, GI Innovation partnered with Samsung Biologics to develop cell lines for clinical sample production of this candidate.
GI Innovation CEO Jang Myung Ho stated that the company’s next-generation dual fusion candidate represents the culmination of proactive mechanism design aligned with allergy treatment trends, years of accumulated research, and AI-driven optimization. He noted that it has become one of the company’s most advanced assets outside its immuno-oncology pipeline and emphasized a commitment to rapidly demonstrating its differentiated mechanism and efficacy through clinical development.
