GE HealthCare Korea announced on Monday that its Surgical Pleth Index (SPI), a monitoring tool that quantifies patients’ real-time pain responses during surgery, has been officially recognized as a new medical technology. This recognition follows a safety and efficacy evaluation by the Korea Health Industry Development Institute.
The SPI is now classified as an independent technology capable of quantitatively assessing pain responses to surgical stimuli in patients under general anesthesia, paving the way for its use in Korean medical settings.
The evaluation of new medical technologies is conducted in accordance with healthcare regulations to safeguard public health and drive medical advancements. A dedicated committee within the Department of Health and Human Services assesses the safety and efficacy of new medical technologies based on scientific evidence, with the results announced by the Secretary. GE HealthCare Korea’s SPI received official approval on December 29 last year.
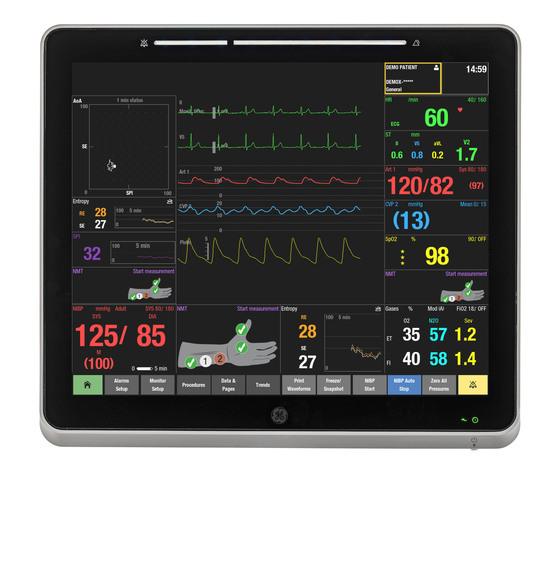
SPI analyzes autonomic nervous system responses using fingertip plethysmography data, providing a single indicator that quantifies both patient pain responses and analgesic effectiveness.
This allows healthcare providers to more intuitively and accurately gauge changes in patients’ pain responses, enabling quick adjustments to pain medication timing and dosage.
Traditionally, patient pain assessment has relied on indirect hemodynamic indicators like blood pressure and heart rate. GE HealthCare’s SPI offers a novel approach by continuously and quantitatively monitoring pain responses through autonomic reactions in peripheral blood flow.
This new medical technology classification addresses the clinical need for more objective, quantitative pain-response assessments in healthcare settings.
Furthermore, SPI is a key component of GE HealthCare’s Anesthesia Optimization (AoA) framework, which comprehensively evaluates consciousness levels, amnesia, analgesia, muscle relaxation, and autonomic stability. SPI works alongside other indicators, such as entropy, neuromuscular monitoring (NMT), and hemodynamic variables, to provide visual information on patient status, supporting clinical decision-making.
Kim Yong Deok, CEO of GE HealthCare Korea, said the recognition of SPI as a new medical technology was a significant milestone that officially validated its clinical safety and effectiveness as a pain response assessment tool. He added that he anticipated this would enhance patient safety and improve the precision of anesthesia and pain management, and said GE HealthCare remained committed to advancing care environments and treatment outcomes for both patients and healthcare professionals.



