The world’s first allogeneic umbilical cord blood-derived stem cell treatment, Cartistem®, is making significant strides in its global expansion. Medipost, a pioneer in stem cell therapy in South Korea, has ramped up its efforts by conducting an intensive training program for U.S. experts.
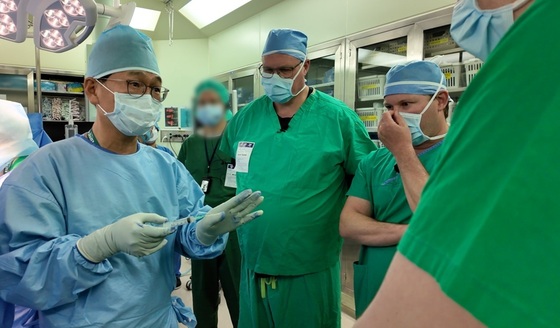
Medipost announced Tuesday that it held a Train the Trainer Program on April 23 and 24. The program invited key opinion leaders from the U.S. in preparation for Cartistem®’s entry into Phase 3 clinical trials in the country.
This initiative aimed to deepen understanding of Cartistem®’s surgical methods and overall clinical procedures among participating U.S. medical professionals.
The training attracted prominent orthopedic experts from across the U.S., including Dr. Andreas Gomoll from the Hospital for Special Surgery in New York, Dr. David Flanigan from Ohio State University, Dr. Kenneth Zaslav from Northwell Health, and Dr. Seth Sherman from Stanford Health Care.
Professor Jae Du Yoo from Ewha Womans University Mokdong Hospital was an advisor who guided participants through various aspects of the treatment. These included Cartistem® product education, patient profiling and selection protocols, surgical approaches and techniques, postoperative care and follow-up procedures, and clinical case reviews.
Yoo’s insights on the practical application of Cartistem®, including its mixed administration and precise delivery methods, were particularly well-received by attendees.
The program facilitated a robust exchange of clinical insights through hands-on training, surgical demonstrations, and in-depth discussions between U.S. and Korean medical professionals, fostering stronger global medical collaboration.
In an interview, Dr. Gomoll highlighted the treatment’s advantages, noting that Cartistem patients recover more quickly than those undergoing traditional therapies. He added that the treatment promotes cartilage regeneration and significantly reduces inflammation across the entire joint.
He added that the extensive long-term clinical data accumulated over Cartistem’s decade of use in South Korea offers considerable reassurance for American patients. He emphasized that this data will play a crucial role in supporting the upcoming Phase 3 trials in the U.S.
Seung Jin Lee, head of Medipost’s Global Business Division and co-CEO of its U.S. subsidiary, affirmed its commitment to accelerating clinical trials and expanding its infrastructure to penetrate global markets.
Medipost has scheduled a second Train the Trainer Program for key U.S. orthopedic experts in May, further solidifying its position in the U.S. medical landscape.



