
Digmbio, having secured 12 billion KRW (approximately 8.7 million USD) through Series A funding, is poised to accelerate clinical trials for its next-generation cancer drug candidate and preclinical studies for a treatment for degenerative brain disease.
Patient Recruitment for Phase 1 Trial of DM5167 Targeting Solid Tumors Progresses Well
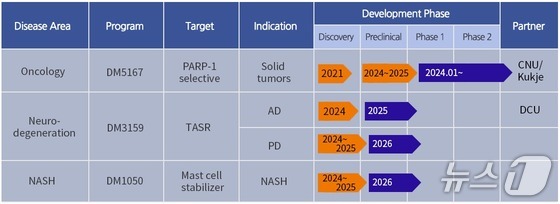
Industry sources reported on Friday that Digmbio is currently recruiting patients for the Phase 1 clinical trial of DM5167, its second-generation PARP-1 selective inhibitor candidate.
This study will involve 76 patients with advanced solid tumors to evaluate the safety, tolerability, efficacy, and pharmacokinetic properties of DM5167. The trial, which began last year with patient recruitment starting in October, is expected to conclude in August 2026.
The Phase 1 trial for DM5167 will be conducted at four major medical institutions in South Korea, including Seoul National University Hospital, Seoul National University Bundang Hospital, Yonsei University Severance Hospital, and Samsung Medical Center. Participating patients will take DM5167 orally every morning on an empty stomach in 28-day cycles.
DM5167 is a second-generation targeted cancer drug candidate that selectively inhibits PARP-1. Digmbio expects DM5167 to effectively overcome the blood toxicity issues associated with existing first-generation PARP inhibitors.
Last October, Digmbio was selected for the Clinical Phase 1 support program by the Korea Drug Development Fund (KDDF), receiving funding for research and development (R&D) costs related to the DM5167 Phase 1 trial.
Digmbio plans to develop DM5167 as a best-in-class cancer drug, aiming to secure proof of concept (POC) data during Phase 1 before pursuing technology transfer agreements with global pharmaceutical companies.
Kim Jeong Min, CEO of Digmbio, stated that if they achieve positive results in Phase 1, they believe technology transfer will be possible, adding that successfully completing Phase 1 is a critical development milestone for Digmbio.
Aiming to Complete Preclinical Trials for DM3159, a Degenerative Brain Disease Candidate
In March this year, Digmbio raised 12 billion KRW (approximately 8.7 million USD) through Series A funding. Investors included Partners Investment, Meritz Securities, MedytoxVenture, Samho Green Investment, Industrial Bank of Korea, CornerStone Partners, and Myriad Life Sciences.
With this funding, Digmbio is conducting DM5167 clinical trials and advancing the development of DM3159, a treatment for degenerative brain diseases.
DM3159 is a novel drug candidate targeting the taste receptor “TASR GPCR.” It has shown neuroprotective and regenerative effects in various degenerative brain disease models, including Alzheimer’s, based on a mechanism of action distinct from existing treatments.
Digmbio reported significant cognitive function improvements in Morris water maze behavioral experiments using a 3xTG dementia animal model. The company is currently conducting scale-up research for nonclinical toxicity testing.
Founded in July 2020, Digmbio brings together experts in new drug R&D. CEO Kim Jeong Min, who has worked at LG Chem, GC Biopharma, and Jeil Pharmaceutical, is joined by Dr. Kang Jae Hoon, former head of research at Il Dong Pharmaceutical, as Chief Technology Officer (CTO), and Dr. Kim Jung Ho, former vice president at Oscotec, as head of research. Together, they are driving the development of Digmbio’s pipeline.



