
Research data indicates that ID110521156, an oral glucagon-like petitide-1 (GLP-1) obesity drug candidate, is showing promising efficacy with minimal side effects. It is expected to be considerably more affordable than existing treatments and aims to dominate the market as a best-in-class option.
Lee Chae Joon, President and Chief Operating Officer (COO) of Ildong Pharmaceutical (Ildong), told News1 that given the proven safety profile of ID110521156, its potential extends beyond daily medication. He noted that they are exploring various combination therapies and even considering an expansion into metabolic disorders such as fatty liver disease (MASH).
ID110521156 Poised to Shake Up the Divided Obesity Treatment Market
Industry experts predict that the GLP-1 obesity treatment market, known for drugs like Wegovy, will be split between long-acting injectables administered monthly and oral medications. Currently, most injectables require weekly dosing.
GLP-1 obesity treatments are receptor agonists (RAs) that mimic the GLP-1 hormone. This hormone, released after eating, regulates insulin secretion and suppresses appetite.
Lee noted that all GLP-1 drugs have side effects, including muscle loss accompanying weight reduction, liver toxicity, and gastrointestinal issues. Further noting that the key is to find the right dosing strategy to manage these effects effectively.
Clinical trials of existing GLP-1 obesity treatments have shown side effects such as nausea, vomiting, and diarrhea in some patients. Although generally mild, these effects can cause discomfort after administration.
Lee anticipates a dual approach: using injectables for severe obesity cases, where some side effects might be tolerable in exchange for effective treatment, and daily oral medications for those focused on prevention and long-term management.
He believes that ID110521156, developed by Ildong’s research and development (R&D) subsidiary Yunovia, is well-positioned to succeed in this evolving market.
At the American Diabetes Association (ADA) meeting last June, Yunovia presented promising Phase 1 results for ID110521156. The drug demonstrated significantly fewer gastrointestinal side effects across its effective dose range compared to existing GLP-1 RAs, showcasing excellent tolerability.
Yunovia also reported impressive efficacy in reducing blood sugar levels and body weight. In the 100 mg (about 0.00353 oz) dosage group, participants averaged a 6.9% weight loss over four weeks, with some losing up to 11.9%. Notably, 66.7% of subjects in this group lost more than 5% of their body weight, compared to 0% in the placebo group.
Lee emphasized that the Phase 1 results were crucial. He noted that they observed a 4-5% weight loss in just four weeks at low doses, with some participants experiencing up to a 7-8% loss. Further highlighting that this candidate is highly competitive, and its favorable side effect profile might allow for even lower, safer dosing.
ID 4.0: Driving Management and R&D Innovation
The development of next-generation drugs like ID110521156 is part of Ildong’s broader ID 4.0 management innovation strategy.
ID 4.0 aims to create competitive advantages through two key policies: boosting revenue and profits, and securing new growth drivers while building a sustainable business framework.
This strategy marks Ildong’s fourth major transition, following its founding (ID 1.0), overcoming the International Montary Fund (IMF) crisis (ID 2.0), and restructuring into a holding company (ID 3.0).
Lee explained, “ID 4.0 is about instilling a ‘winning spirit’ throughout the company. We are setting specific performance targets and initiatives, and are committed to continuous improvement until we achieve our goals.”
Ildong Pharmaceutical, the core company of the group, reported sales between 561 billion and 635.8 billion KRW (about 401.1 million to 454.6 million USD) from 2020 to 2022. During this period, it invested over 400 billion KRW (about 286 million USD) in R&D. Although this led to operating losses until 2023, the company returned to profitability last year after spinning off its R&D division.
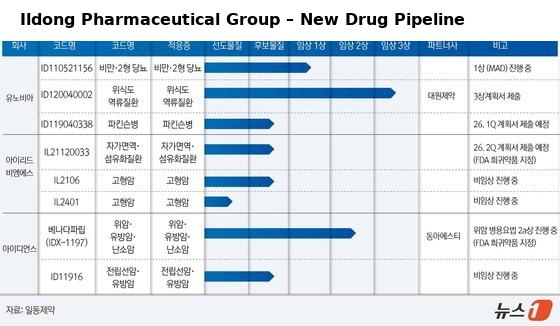
Beyond ID110521156, Ildong’s pipeline includes the Potassium-Competitive Acid Blocker (P-CAB) peptic ulcer treatment ID120040002, the Parkinson’s drug ID119040338, and the autoimmune therapy IL21120033.
The company has also ventured into new modalities with IL2112 for pulmonary fibrosis and heart disease, and IL2106, a targeted protein degradation (TPD) molecular glue.
Lee stated that it expects to focus on technology transfers for the next 10-20 years while building our global commercialization capabilities. Further noting that developing innovative drugs is challenging, but they are conducting R&D with clear commercial potential in mind. Finally, he noted that through ID 4.0, they are embarking on an exciting growth journey, persistently pushing forward until it succeeds.
Lee, President and COO of Ildong Pharmaceutical – Key Career Highlights
△ Bachelor and Masters in Mechanical Engineering, University of British Columbia, Canada △ Ph.D. coursework in Biomedical Engineering, Northwestern University, U.S. △ Executive Master of Business Administration (MBA), University of Michigan, U.S. △ Senior Consultant, Pharmaceuticals and Consumer Sector, A.T. Kearney, Chicago Headquarters △ Head of Global Marketing Operations Strategy and Marketing, Samsung Electronics △ Executive Director, Business Development and Strategy, GSK Asia Pacific △ Senior Executive Director, Global Business Division, Dong-A ST △ Chief Executive Officer (CEO), Yungjin Pharmaceutical △ Current COO, Ildong Pharmaceutical △ Current CEO, Yunovia and iLead BMS (Ildong Pharmaceutical Group subsidiaries) △ Current Chief Business Officer (CBO), Idience (Ildong Pharmaceutical Group)



